Mutagens
Gentronix offers a range of screening options to help our clients assess the mutagenic potential of their test substances, using small amounts of test item and delivered on responsive study timelines.
SERVICE INFORMATION
Due to the strong association between point mutations and carcinogenicity, companies developing new substances incorporate gene mutation assessment into their screening at an early stage, both through in silico structural alert software, and in vitro testing strategies. Identifying mutagenic liabilities early in a project, enables chemistry to be adapted to remove the mutagenic liability, or focus to be shifted away from a challenging scaffold.
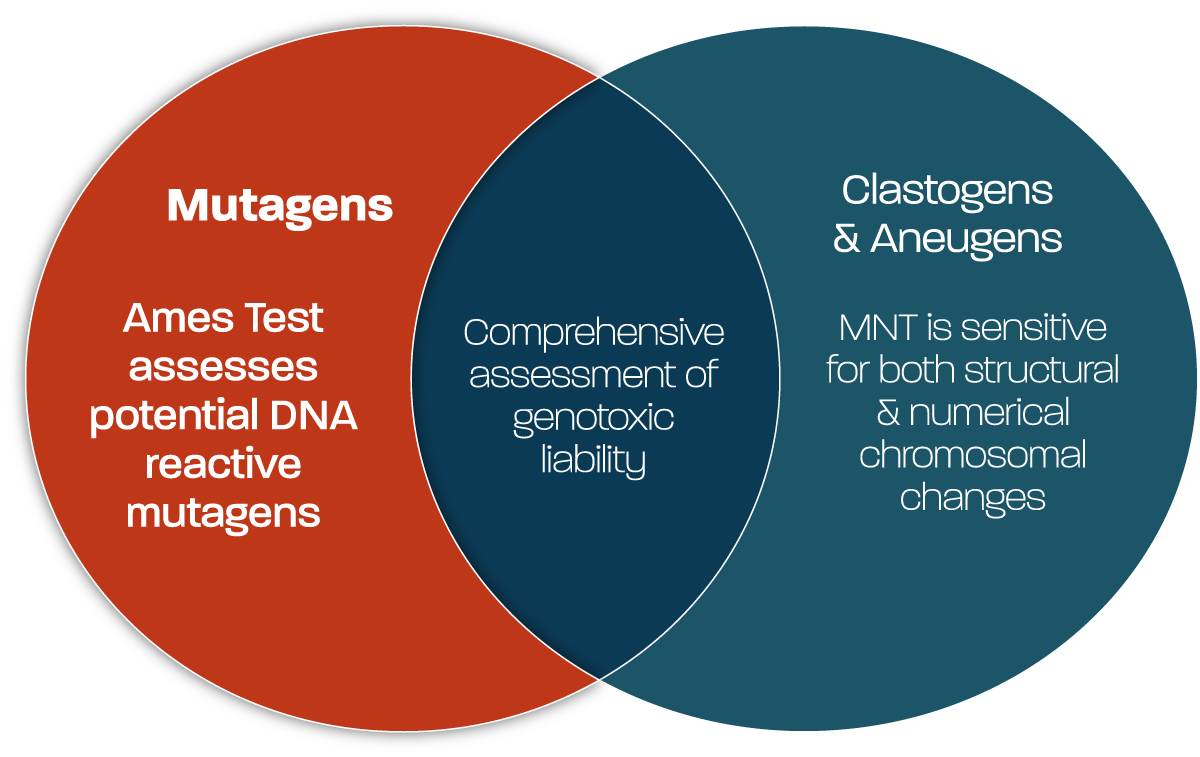
Across global industries, the Ames test is the gold standard in vitro test for assessing mutation hazards. It forms an essential component of the regulatory testing battery due to its ability to detect DNA-reactive substances capable of inducing point mutations. The assay uses a variety of Salmonella or E. coli bacterial strains, which are engineered to carry site-specific mutations leading to auxotrophy for amino acids histidine or tryptophan (E. coli). When exposed to a mutagenic test substance, these strains can return to prototrophic status, forming colonies in the absence of supplemental amino acids. These ‘reverse mutant’ colonies can then be counted against a spontaneous reversion background, and the mutagenicity frequency and potential of test material can be determined.
Whilst the OECD 471 guideline Ames test enables a comprehensive assessment of mutagenic risk, the many agar plates and test strains, it requires results in a test substance requirement of gram quantities not often available in early discovery and an assay format that is not conducive to screening multiple compounds. To address these challenges whilst still retaining accurate prediction of the OECD 471 guideline Ames test, Gentronix offers a range of screening options that enable the detection of mutagens in assay formats capable of screening 100s of compounds from as little as 10 mg of test substance: