The Challenge for the Food Industry
Food contact materials (FCMs) come in many forms, be it coffee cups, Styrofoam containers, cans etc. Not only do FCMs themselves need to be assessed for safety, but the food industry is increasingly required, particularly under the European regulatory framework, to assess the safety of non-intentionally added substances (NIAS), which potentially migrate into food.
FCMs are typically stable materials. Otherwise, they would be unable to perform their intended function – protecting food. However, food may be in contact with FCMs for a long time; for instance, this could be for years for tin cans. To simulate the migration of intentionally added substances (IAS) and NIAS into food, migration experiments are performed, essentially solvent extractions under exaggerated conditions. These migrations can then be used to identify IAS and NIAS for safety assessment or for performing bioassays on the migration itself as part of the toxicology characterisation.
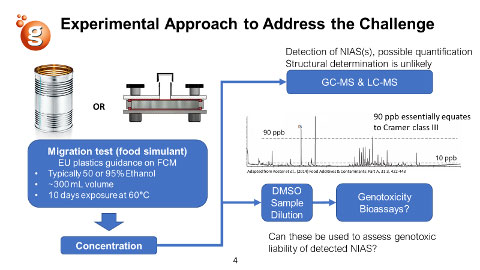
However, NIAS are typically present at low concentrations, and even identifying them presents a significant challenge. Also, the risk assessment of NIAS has proven difficult due in part to questions regarding the sensitivity of test methods and the small amount of migration available for toxicological testing. A degree of pragmatism is required, as testing, every substance from migration is not feasible. One approach the industry proposed is applying the threshold of toxicological concern (TTC) Cramer class III threshold set at 90 ppb. However, this assumes a lack of direct DNA reactivity (often detected in the Ames test) of substances in the migration sample. So what if you perform mutagenicity testing on the migration sample? Thus, the question is whether conventional genotoxicity assays are sufficiently sensitive to detect genotoxicity at very low doses.
The utility of the Ames test
For decades, the Ames test (OECD guideline 471) has been a mainstay of genotoxicity assessments. When conducted in combination with the in vitro micronucleus, there is the ability to detect point mutations, clastogens and aneugens. The Ames test uses several strains of bacteria (Salmonella typhimurium or E. coli are the test organisms used) with mutations at specific loci. When grown on agar plates with minimal amino acid histidine (or tryptophan for E. coli), only mutated bacteria will have regained the ability to grow once the histidine has been depleted. Therefore, an increase in the number of grown colonies indicates that the tested chemical causes mutations in this test system and warrants further investigation.
The Ames test typically detects point mutations, which are particularly relevant to safety assessment given they often result from direct interactions with DNA, meaning they may have a linear dose-response and can be very potent.
Determining the lowest effective dose in the Ames test
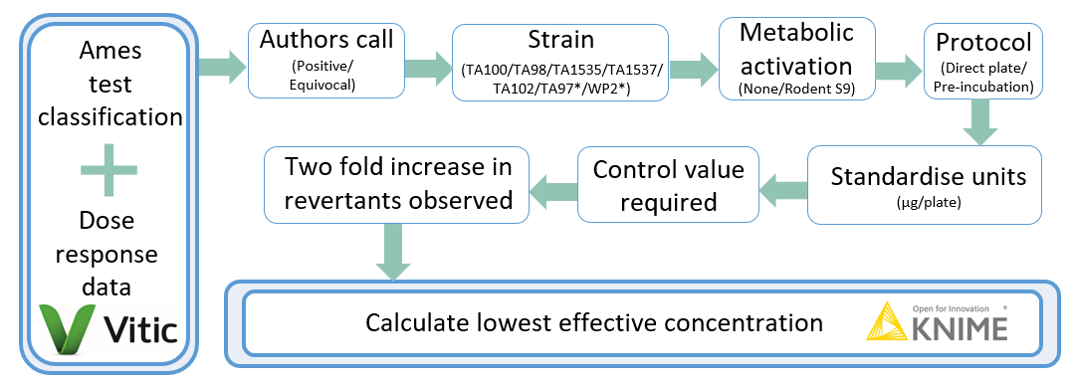
In collaboration with Lhasa Limited, we examined the sensitivity of the Ames test – the lowest dose at which substances were positive – and compared this to the potential threshold concentrations relevant to NIAS safety assessment. We were asking how many of these substances would be expected to be detected as positive in an Ames assay FCM migration sample if present at regulated limits (e.g. at 90 ppb or the Cramer class III TTC threshold). What confidence level could be placed in a negative Ames test on a migration sample?
To do this, Lhasa interrogated their Vitic database, which is collated from publicly available Ames test dose-response data. Not all Ames tests are conducted in the same manner, but a standardised approach is provided by OECD test guideline 471, which is intended to provide a robust study design suitable for regulatory assessments. We used these principles to eliminate study records that did not meet quality criteria, such as studies that were not conducted with the standard tester strains or were not considered positive by the study’s authors. The criterion for an Ames positive was any experimental run in a tester strain where the number of colonies was 2-fold or greater than that of the concurrent solvent control.
Conclusions
The full details of the analysis will not be gone into here, as we are looking to publish them in a scientific journal in the near future; here are some key conclusions.
More than 1,200 substances have been collated from the Vitic database. Data analytics have been applied to determine the lowest dose and the fold increase at which a mutagenic response could be detected. The lowest dose for an Ames positive test was one ng/plate for this data set and showed the Ames test could detect potent mutagens. Using the 10ppb limit set by the food contact industry (derived from regulations specific to food contact plastics), the Ames test theoretically detected 11.3% of substances in this dataset. We also investigated the lowest effective doses in mammalian genotoxicity assays in vitro. However, no assay was more sensitive than the Ames test when judged by the lowest effective doses.
Gentronix has considerable experience conducting and interpreting Ames tests and provides them in various formats to suit all regulatory and early research screening needs, including tests that only require tens of milligrams of material. If you would like to know more, please get in touch with paul.rawlinson@gentronix.co.uk or info@gentronix.co.uk.
See here for more details on our regulatory Ames studies and screening options.
Gentronix conducted this work in collaboration with Grace Kocks and Richard Williams at Lhasa. Tragically, Rich passed away earlier this year and is greatly missed.