In Vitro Skin Sensitisation Assays
As described in a previous blog, the prediction of skin sensitisation is an important part of risk assessment for many types of chemicals. Historically, skin sensitisation potential has been assessed using in vivo models. However, regulatory requirements and animal welfare concerns have prompted a concerted effort to replace animal testing for this regulatory endpoint. Against this background, the detailed mechanistic understanding of the biological and chemical events leading to the induction of skin sensitisation as described in the appropriate adverse outcome pathway (AOP) has enabled the development of many non-animal test methods aligned to the early key events.
Accurate prediction of skin sensitiser potency continues to be challenging (although options such as GARD may prove useful), but there has been good progress in using in vitro and in chemico approaches to predict hazards. These tools, therefore, are very useful for replacing (or partly replacing) animal methods when complying with classification and labelling requirements. Positive results at present may require follow-up with a local lymph node assay to discriminate between GHS category 1A and 1B.
Each of these in vitro test methods aims to quantify the impact of a substance on one or more of the key events as outlined in the AOP to predict sensitising potential. To date, six non-animal methods addressing the first three key events of skin sensitisation have undergone full validation and have been translated into internationally recognised OECD Test Guidelines.
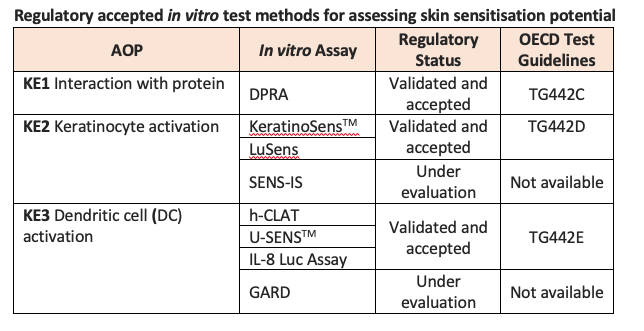
The only test method to have completed validation and achieved regulatory acceptance so far for KE1 is the Direct Peptide Reactivity Assay (DPRA), an in chemico test method that addresses the molecular initiating event of the AOP by measuring binding to skin proteins using model synthetic peptides. This test method measures cysteine and/or lysine depletion using High-Performance Liquid Chromatography (HPLC).
For KE2, two in vitro cell-based test methods have reached regulatory acceptance: KeratinoSensTM and LuSens. These assays, described in OECD TG442D, are based on the understanding that skin sensitisers are known to induce genes regulated by the antioxidant response element (ARE) in keratinocytes. These in vitro assays use luciferase induction to measure the Nrf2-mediated activation of ARE-dependent genes in human keratinocyte-derived cell lines comprising a luciferase reporter gene. SENS-IS, a third assay aimed at addressing KE2, differs from KeratinoSensTM and LuSens in that it uses Reconstructed human Epidermis (RhE) and is a gene-expression-based test method that seeks to discriminate between irritants, non-sensitisers and sensitisers, and to provide a correlate of potency. SENS-IS is currently under evaluation and discussion.
Three fully validated in vitro test methods addressing KE3 (DC activation) have been adopted by the OECD (OECD TG442E): the human Cell Line Activation Test (h-CLAT), U-SENSTM and the IL-8 Luc Assay. All three assays measure biomarkers of DC maturation using DC surrogates. Briefly, h-CLAT measures changes in cell surface expression of CD86 and CD54 using a human monocytic leukaemia cell line (THP-1 cells), U-SENSTM quantifies increases in CD86 on the cell surface of a human histiocytic lymphoma cell line (U937 cells). The IL-8 Luc Assay measures luciferase gene induction as an indicator of IL-8 activity in a THP-1-derived IL-8 reporter cell line. A fourth assay, the Genomic Allergen Rapid Detection (GARD) assay, uses a human myeloid cell line derived from MUTZ-3 to predict sensitising potential and potency based on gene expression profiling and is currently under evaluation.
There are currently no accepted non-animal methods for KE4.
IATA and DA
Each of the test methods described above, when used individually or in combination, has its own set of limitations. For example, DPRA lacks a metabolic component and is unsuitable for metals which bind to proteins via mechanisms other than covalent binding. Cell-based assays are unsuitable for cytotoxic compounds, cell stressors, or materials with solubility issues. KeratinoSensTM is incompatible with chemicals that interfere with luciferase activity or with exclusive reactivity towards lysine, while h-CLAT cannot be used for strongly fluorescent compounds or substances with log Kow above 3.5.
These limitations, combined with the complexity of the underlying biology of skin sensitisation, indicate that no single assay can predict skin sensitising potential. The general assumption is that only a combination of methods in an Integrated Testing Strategy (ITS) will replace the use of animals for skin sensitisation testing.
Consequently, it is believed that data derived from the validated assays aligned to the first three key events of the skin sensation AOP should be considered in combination with other relevant information such as structural alerts and read-across from chemical analogues, in the context of an Integrated Approach to Testing and Assessment (IATA). The overall assessment within an IATA follows an iterative approach and is based on the weight of evidence (WoE), implying the involvement of expert judgment. As a result, many testing strategies that do not require expert judgment and are rule-based, consisting of fixed Data Interpretation procedures (DIP), have been proposed.
Conclusions
The current battery of non-animal test methods for skin sensitisation can support the discrimination between skin sensitisers and non-sensitisers when applied in the context of IATAs for hazard identification. Our understanding of the biology underlying skin sensitisation is excellent, but the challenge is how to utilise information from the accepted assays for regulatory decision-making and which emerging assays might inform potency. Significant advances are being made towards the long-term goal of making better predictions without using animals.
At Gentronix, we offer GLP DPRA, KeratinoSensTM and h-CLAT studies to include as part of your testing strategy for assessment of the first three key events of skin sensitisation AOP – allowing you to assess your test chemical for these properties without needing to use an animal model. We are working with collaborators on research into chemical mixture assessment in these systems, which we see as the next challenge for this area.








